Palladium membrane purification
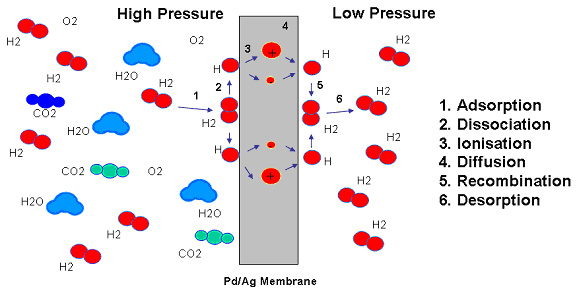
How Palladium Membrane Purifiers WorkPalladium membrane hydrogen purifiers operate via pressure driven diffusion across palladium membranes. Only hydrogen can diffuse through the palladium. The palladium membrane is typically a metallic tube comprising a palladium and silver alloy material possessing the unique property of allowing only monatomic hydrogen to pass through its crystal lattice when it is heated above nominally 300░ C. The hydrogen gas molecule coming into contact with the palladium membrane surface dissociates into monatomic hydrogen and passes through the membrane. On the other surface of the palladium membrane, the monatomic hydrogen is recombined into molecular hydrogen - the ultrapure hydrogen used in the semiconductor process.
Palladium purifiers provide <1 ppb purity with any inlet gas quality. Impurities removed include O2, H2O, CO, CO2, N2 and all hydrocarbons (THC) including methane (CH4). Maximum operating pressure is 250 psig at 300 to 400░C; high pressure vessels can be designed as well. Normal life expectancy of a palladium membrane purifier is 5 years and no routine maintenance required.
Palladium Membrane Purification - Development History and Mechanical Design Issues (1)
The selective diffusion of hydrogen through palladium membranes has been the subject of extensive investigation since its discovery by Sir Thomas Graham in 1866. Most of this work was of a scientific or theoretical nature with little or no effort being made to make use of this phenomenon on anything other than a laboratory scale. The discovery of the unique properties of a palladium alloy has made possible the utilization of this selective action for the industrial production of ultrapure hydrogen. Large size diffusion cells have become available and have been used successfully in plant operations.
At or near ambient temperature, palladium metal exhibits a remarkable property of dissolving very considerable volumes of hydrogen. Sir Thomas Graham's early work showed after degassing in a vacuum, a piece of palladium foil selectively dissolved hydrogen to 526 times its own volume on cooling from 245░ C to room temperature in hydrogen.
When the temperature of a hydrogen saturated specimen is increased, the dissolved gas is expelled. If a suitable hydrogen partial pressure difference exists across the metal membrane, hydrogen permeation takes place and increases with increasing temperature. Below the critical temperature Tc = 300░ C and the critical pressure of Pc = 20 atm, there exists a region over which the hydrogen pressure is invariant with changing hydrogen concentration. This is the coexistence of two solid phases referred to as the a and ▀. On repeated heating and cooling, an irreversible change takes place in the lattice structure.
This destructive structural change resulting from a - ▀ phase cycling has been the principle deterrent to the industrial development of palladium membranes for the separation and/or purification of hydrogen. This effect can be avoided or minimized by maintaining the palladium surface at the temperature above the critical (Tc = 300░ C) as long as it is in an atmosphere of hydrogen. A more generally satisfactory answer to the problem has resulted from the discovery of the unique properties of a silver-palladium alloy. The addition of silver to the palladium has been found to impart a marked stabilizing effect to the metal structure. Alloy compositions containing 20% or more silver show none of the damaging effects of a - ▀ phase cycling.
The Mechanism of Hydrogen Diffusion
The mechanism of hydrogen involves a series of steps: (1) adsorption, (2) dissociation, (3) ionization, (4) diffusion, (5) reassociation and (6) desorption. Several molecules of hydrogen and nitrogen atoms are on the metal surface. Within the metal, hydrogen loses its electron to the palladium structure and diffuses through the membrane as an ion (or proton). At the exit surface the reverse process occurs. Various examples of experimental evidence have been advanced to substantiate this mechanism.
Only hydrogen appears to possess the ability to diffuse through palladium or palladium alloys. Assuming no pinholes or micro-cracks, the hydrogen issuing from the low-pressure side of a membrane may be looked upon as a standard of absolute purity. Attempts to detect the presence of impurities show only traces in the parts-per-billion range. These trace impurities, if indeed there are any, probably reflect either mechanical defects or incomplete outgassing of the walls of the downstream of parts of the system itself.
Poisoning
The hydrogen transfer process is affected by the presence of contaminants in the feed gas which is not surprising as the palladium alloy surface is catalytic in nature.
There are two ways poisoning can occur. The first is a chemical reaction with the surface such as occurs with gases containing sulfur. At elevated temperatures, sulfur attack is evidence by a discoloration of the surface and a rapid disintegration of the metal structure. The extent to which sulfur attack can occur and still allow regeneration has not been established. Best practice calls for maximum removal of sulfur compounds ahead of the diffusion cell.
The second way poisoning can happen is from the chemisorpotion of unsaturated hydrocarbons. Regeneration is readily accomplished by the brief exposure of the contaminated surface to air at the operating temperature (800░ F). Evacuation of the system, both before introducing air and again before reintroducing hydrogen, is an important safety requirement.
Other gases and vapors show effects ranging from partial poisoning to no effect at all other than a lowering of the hydrogen partial pressure. Under conditions of the test, water vapor, ammonia, carbon dioxide and carbon monoxide do not appear to poison the surface. The drop in transfer rate is due to the partial pressure effect of a 50/50 gas mixture. As soon as the impurity gas is turned off, the transfer rate immediately returns to normal.
Saturated hydrocarbons, methanol, benzene, and cyclohexane vapors mixed with hydrogen also appear to have no permanent effect on hydrogen transfer. Mercury, chlorine and hydrogen chloride are reported to be surface poisons. Chlorine or hydrogen chlorine in the presence of iron forms an iron chloride which reduces hydrogen transfer by depositing on the diffusion surface.
Design
For maximum hydrogen transfer there are many considerations in the design of a diffusion cell. It is generally desirable to produce a maximum diffusion area within a given cell volume to conserve space. Next the membranes, or transfer area, should be as thin as possible consistent with the pressure requirements of the system. Of course the design must be reasonable to manufacture at a reasonable price.
A type of cell that is commercially available has a bundle of small diameter, thin-walled palladium alloy tubes manifolded together at the open end and having their opposite ends closed. This tube bundle is contained within a close-fitting, heavy-walled, stainless steel shell with inlet and outlet connections for feed gas, bleed and ultrapure hydrogen. The tubes are 0.063" O.D. with a 0.003" wall. Manifolding the tubes together at only one end makes possible close packing that results in a maximum surface area in a minimum volume. Fixing only one end also allows for tube elongation to minimize the build up of internal stresses or strain within the tubing or at the manifold junction.
Feed gas enters at one end of the cell and flows over the outside of the tube bundle. The bleed gas containing all of the non-diffusible components leaves the cell at the bottom. Ultrapure hydrogen (UPH) diffuses through the walls of the tubes and is collected in a common manifold at the top of the cell. This design provides rugged construction and is easily incorporated into any commercial hydrogen system.
For the purification and/or separation of hydrogen from gas mixtures, the palladium alloy system offers a number of attractive features; the foremost of these is the quality of the ultrapure hydrogen itself. The diffusion cells involve no moving parts and the intrinsic value of the noble metal represents a recoverable investment.
The feed gas containing hydrogen enters a preheat zone through a pressure reducing valve and flows to the inlet of the diffusion cell. The bleed gas leaves the bottom of the cell through a heat exchanger (cooler). A bleed control valve and flow meter provide the necessary bleed gas control. The ultrapure hydrogen leaves the cell by the way of the heat exchanger and through a UPH control valve.
While the use of palladium-silver alloys prevents the destructive a - ▀ phase cycling, damage to the membrane, particularly the joints where the palladium-silver tubes are connected to the manifold, can occur if the membrane is subjected repetitive temperature cycling in a hydrogen environment. To minimize this occurrence, removing the hydrogen prior to cooling the membrane is recommended. This can be accomplished by the use of vacuum to evacuate the hydrogen from the cell prior to cooling it down. Another method involves purge the feed side of the membrane with an inert gas or nitrogen. This will remove the hydrogen from the feed side of the membrane. When the partial pressure of hydrogen on the pure side of the membrane exceeds that of the feed side, the hydrogen will "back-diffuse" from the pure side to the feed side of the membrane and will be swept out of the cell by the purge gas. Once the hydrogen is removed, the membrane can be cooled down.
Conclusion
The discovery of the stability of palladium-silver alloys and the ability to manufacture membranes of these alloys made hydrogen purification using palladium-silver membranes possible. Palladium-silver membrane based hydrogen purification is used in many applications including semiconductor manufacturing. In particular, these membranes have wide spread use in the compound semiconductor industry, where the absolute purification of hydrogen is critical. When coupled with an effective purge system, a palladium-silver membrane based hydrogen purifier will give years of reliable service.
References:
1) Dr. J.B. Hunter, "Ultrapure Hydrogen By Diffusion Through Palladium Alloys", Presented at the Symposium on the Production of Hydrogen Petroleum Division, The American Chemical Society Meeting, New York, Fall 1963.




 BACK TO PURIFICATION TECHNOLOGIES
BACK TO PURIFICATION TECHNOLOGIES